Chapter 11 Stoichiometry
Chapter 11 - Stoichiometry STUDY PLAY reactant the starting substance in a chemical reaction stoichiometry the study of quantitative relationships between the amounts of reactants used. The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project the UC Davis Office of the Provost the UC Davis.

Chapter 11 Stoichiometry Ppt Download
Matter and Change Chapter 11 209 StoichiometryStoichiometry CHAPTER 11 SOLUTIONS MANUAL Section 111 Defining Stoichiometry pages 368372 Practice Problems pages 371372 1.
. Zubaria on July 25 2020 at 817 am. 1 N2 3 H2 2. TeachingLearning includes revision notes for problem solving with 1750 trivia questions.
A reactant that is. A reactant that limits the extent of the reaction and determines the amount of product formed. Particle and Mole Relationships.
Class 11 Chemistry Notes - Chapter 1 - Stoichiometry - Notes. Easy notes that contain overview questions and key points of the chapter. Figure 1141A Flowchart for Stoichiometric Calculations Involving Pure Substances The molar masses of the reactants and the products are used as conversion factors so that you can.
The identity of the reactants helps scientists to predict the products in a chemical reaction. Learn vocabulary terms and more with flashcards games and other study tools. Interpret the following balanced chemical equa-tions in terms of particles moles and mass.
Start studying Chapter 11 Stoichiometry. The join to purchase and make bargains to download and install chapter 11 stoichiometry fittingly simple. STOICHIOMETRY MOLE TO MOLE RATIO When nitrogen and hydrogen gas are heated under the correct conditions ammonia gas NH3 is formed.
Stoichiometry Notes Packet Big Picture Ideas. Chapter 11 - Stoichiometry Lesson 1. In a balanced equation the ratio between the numbers of moles of any two substances.
Chapter 11 Stoichiometry Study Guide Answer Key. Limiting and Excess Reactants The limiting reactant in the reaction limits the extent of the reaction and thereby determines the. The identity of the reactants helps scientists to predict the products in a chemical reaction.
Limiting Reactant Lesson 4. Use this information as a general reference tool to guide you through this unit. Defining Stoichiometry Lesson 1 Worksheet Lesson 2.
A Level Chemistry quick study guide PDF book. Quantitative relationships exist with. Chemistry - Chapter 11 - Stoichiometry Term 1 15 Stoichiometry Click the card to flip Definition 1 15 the study of the Quantitative or measurable relationships that exist in chemical formulas.
Metabolic Engineering George Stephanopoulos. Describe the types of relationships indicated by a balanced chemical equationreactant. 111 Defining Stoichiometry MAINIdeaThe amount of each reactant present at the start of a chemical reaction determines how much product can form.
Chapter-11-stoichiometry-test 22 Downloaded from edocsutsaedu on November 6 2022 by guest NCERT Solutions for Class 11 Chemistry - BYJUS NCERT Solutions For Class 11. Stoichiometry Notes Packet Big Picture Ideas. In a balanced equation the ratio between the numbers of moles of any two.
Based on the law of conservation of mass. Dont hesitate to ask your teacher. Show that the law of conservation of mass is.
_____ is the study of quantitative relationships between the amounts of. STOICHIOMETRY Stoichiometry deals with numerical relationships in chemical reactions and calculating the quantities of substances. Stoichiometric Calculations Lesson 2 Worksheet Lesson 3.
Hareem on April 11 2020 at 1225 pm. Quantitative relationships exist with. Section 111 Defining Stoichiometry Section 112 Stoichiometric Calculations Section 113 Limiting Reactants.
The starting substance in a chemical reactionstoichiometrymole ratiostate.

Chapter 11 Stoichiometry By Sydney Sturgeon

Solution All About Stoichiometry Powerpoint Studypool

Notes Lessonsplansweek21 Week22 Stoichiometry Chapter11 Final Copy Docx Hchem Weeks 21 22 Chapter 11 Stoichiometry Ms E Announcements 1 Chapter 10 Course Hero

Ch 11 The Mole Mr Murry

Cmc Chapter 11

Class 11 Chapter 1 Lect 14 Topic Stoichiometry And Stoichiometric Calculations Youtube

Chemistry Chapter 11 Stoichiometry Stoichiometry Is The Use Of Balanced Equations To Calculate Chemical Quantities Always Balance Every Equation You Ppt Download

Important Questions For Cbse Class 11 Chemistry Chapter 1 Some Basic Concepts Of Chemistry Updated For 2022 23

Ppt Stoichiometry Chapter 11 Powerpoint Presentation Free Download Id 596191
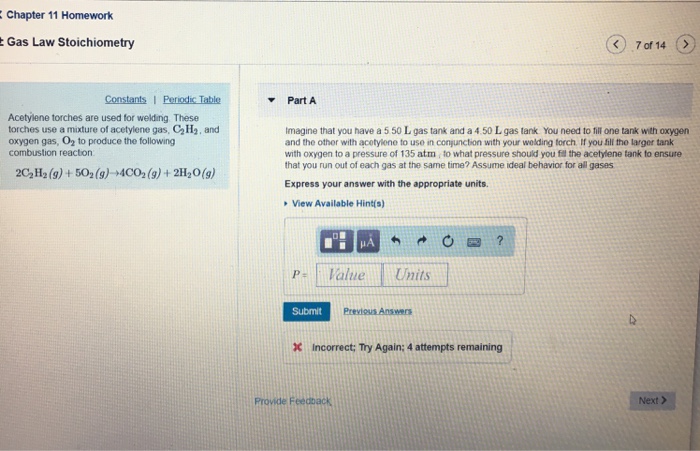
Solved Chapter 11 Homework Gas Law Stoichiometry 7 Of 14 Chegg Com

Stoichiometry Stoichiometry Section 11 1 Defining Solutions Manual Practice Problems Pages Chapter 11 G 2mgo S G 2nh 3 Pdf Free Download

Ppt Chapter 11 Stoichiometry Powerpoint Presentation Free Download Id 4395928

Solved Chemistry 1 Quarter 4 Chapter 11 Class Assignment Chegg Com
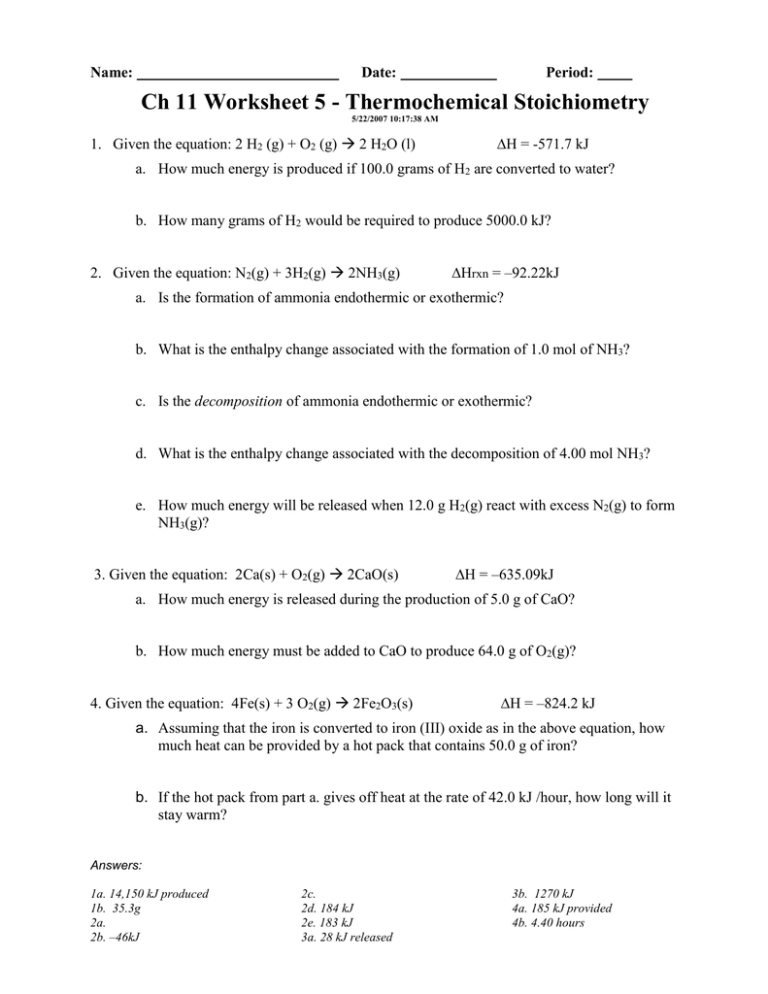
Ch 11 Worksheet 5 Thermochemical Stoichiometry
Stoichiometry Definition

Stoichiometry Stoichiometry Section 11 1 Defining Solutions Manual Practice Problems Pages Chapter 11 G 2mgo S G 2nh 3 Pdf Free Download
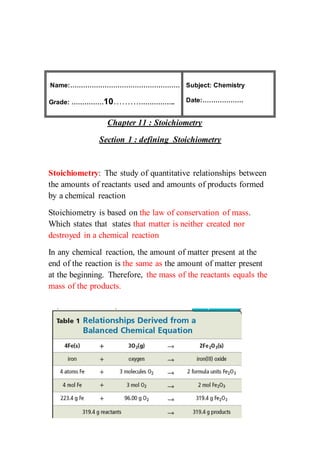
Ch11 Sec 1 Lec Notes